Class XI Biology: Biomolecules
This chapter is where Biology shakes hands with Chemistry. It explains the molecular logic of life—how simple strings of carbon, hydrogen, and nitrogen become the machines (Proteins), the blueprints (DNA), and the fuel (Carbohydrates) of every living cell.
The Chemistry of Life: Mastering Biomolecules
Every living organism is a collection of chemical compounds. If you were to grind up a tissue and analyze it, you wouldn’t just find “life”—you would find a specific ratio of elements. Biomolecules are the organic compounds that perform the heavy lifting inside your cells.
In this chapter, we look at the “Small” (Micromolecules like Amino Acids and Sugars) and the “Giant” (Macromolecules like Proteins, Nucleic Acids, and Polysaccharides) that build the theater of life.
The Core Pillars of Biomolecules
1. Proteins: The Molecular Workhorses
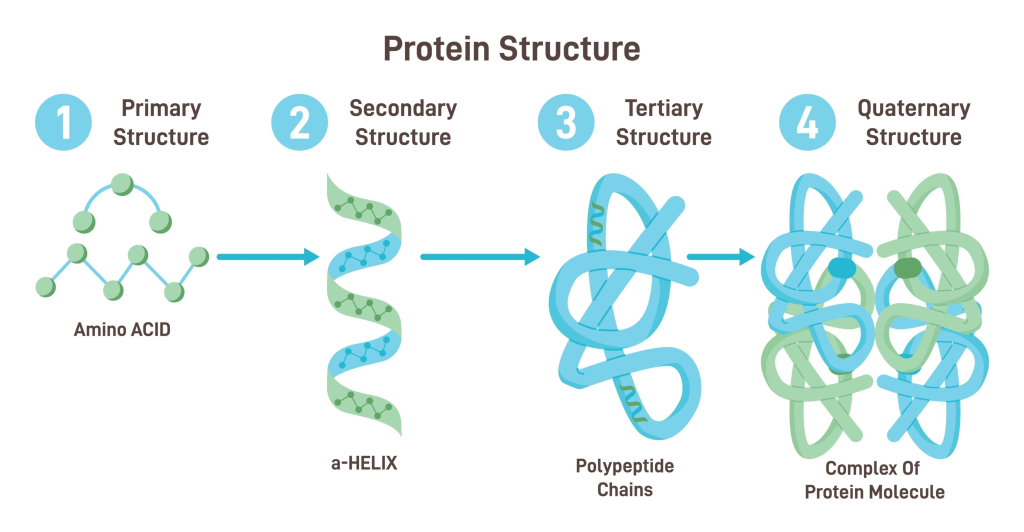
Proteins are heteropolymers of Amino Acids. They are linked by Peptide Bonds.
- Structure: They have four levels of organization: Primary (sequence), Secondary (helices/sheets), Tertiary (3D folding), and Quaternary (assembly of subunits).
- Collagen: The most abundant protein in the animal world.
- RuBisCO: The most abundant protein in the whole of the biosphere.
2. Polysaccharides: The Energy & Structure
These are long chains of sugars (monosaccharides) linked by Glycosidic Bonds.
- Storage: Starch (plants) and Glycogen (animals).
- Structure: Cellulose (plant cell walls) and Chitin (exoskeleton of arthropods and fungal cell walls).
3. Nucleic Acids: The Blueprints
DNA and RNA are polymers of Nucleotides. Each nucleotide has three parts: a Nitrogenous base, a Pentose sugar, and a Phosphate group.
- The Bond: Nucleotides are linked by 3′-5′ phosphodiester bonds.
- The Double Helix: Proposed by Watson and Crick, featuring anti-parallel strands and base pairing (A=T and G≡C).
4. Metabolism and Enzymes
Metabolism is the sum total of all chemical reactions in the body. Enzymes are the biological catalysts that make these reactions happen millions of times faster.
- The “Lock and Key”: Enzymes are specific to their substrates.
- Activation Energy: Enzymes work by lowering the energy barrier required for a reaction to start.
The Gauntlet: 10 Challenging Aptitude Questions
Question 1: The Acid-Soluble Pool
When you grind living tissue in Trichloroacetic acid (Cl₃CCOOH) and filter it, you get two fractions. What are they called, and which one contains the “Biomacromolecules”?
Question 2: The Lipid Paradox
Lipids have a molecular weight of less than 800 Daltons, yet they are found in the “Acid-Insoluble” (Macromolecule) fraction. Why?
Question 3: Amino Acid “Zwitterion”
Amino acids are called “substituted methanes.” Draw or describe the Zwitterionic form of an amino acid. How does it change with pH?
Question 4: Primary Structure Logic
Why is the “Primary Structure” of a protein considered the most important piece of information for a biologist? What does it tell us about the protein’s origin?
Question 5: Reducing vs. Non-Reducing Sugars
In a starch molecule, the “Right end” and “Left end” have different chemical properties. Which end is Reducing and which is Non-reducing?
Question 6: The B-DNA Geometry
In the B-form of DNA (the most common), what is the “Pitch” of the helix, and how many base pairs are found in one complete turn?
Question 7: Essential vs. Non-Essential
What is the difference between an Essential Amino Acid and a Non-essential one? Does our body produce the essential ones?
Question 8: Competitive Inhibition
In the case of the enzyme Succinate Dehydrogenase, the chemical Malonate acts as a competitive inhibitor. How does Malonate stop the enzyme from working?
Question 9: The Michaelis Constant (Km)
In enzyme kinetics, what does a Low Km value indicate about the affinity of an enzyme for its substrate?
Question 10: The Prosthetic Group
Some enzymes require a non-protein “Cofactor” to function. What is the difference between a Prosthetic Group and a Co-enzyme?
Detailed Explanations & Solutions
1. Acid Fractions
The liquid that passes through is the Filtrate (Acid-soluble pool). The part that stays on the cloth is the Retentate (Acid-insoluble pool).
Result: The Retentate contains the Biomacromolecules (Proteins, Nucleic acids, Polysaccharides).
2. Lipid Paradox
Lipids are small molecules, but they are components of cell membranes. When tissue is ground, membranes break into Vesicles (tiny bubbles).
Result: Vesicles are water-insoluble and large, so they get trapped in the filter with the macromolecules.
3. Zwitterion
At a specific pH (isoelectric point), the amino group is positively charged (-NH₃⁺) and the carboxyl group is negatively charged (-COO⁻).
Result: The molecule is electrically neutral but carries both charges.
4. Primary Structure
The primary structure is the linear sequence of amino acids.
Result: It tells us the exact “code” translated from the DNA. Even a single change here can cause diseases like Sickle Cell Anemia.
5. Starch Ends
Result: The Right end is the Reducing end; the Left end is the Non-reducing end.
6. B-DNA Geometry
Result: The pitch is 34 Å (3.4 nm) and there are 10 base pairs per turn. This means the distance between two base pairs is 3.4 Å.
7. Essential Amino Acids
Result: Essential amino acids cannot be made by the body and MUST be taken in through diet. Non-essential ones are synthesized by our cells.
8. Competitive Inhibition
Malonate looks exactly like the real substrate (Succinate). It “tricks” the enzyme and occupies the Active Site.
Result: The real substrate cannot bind, and the reaction stops.
9. Km Value
Km is the substrate concentration at which the reaction velocity is half of Vmax.
Result: A Low Km means the enzyme has a VERY high affinity for its substrate (it doesn’t need much to get to half-speed).
10. Cofactors
Result: A Prosthetic group is TIGHTLY (permanently) bound to the enzyme (like Heme in peroxidase). A Co-enzyme is only LOOSELY/transiently attached (like NAD or Vitamins).
Pro-Tip: The “Polymer” Rule
- Proteins: Heteropolymers (always different amino acids).
- Polysaccharides: Can be Homopolymers (like Cellulose/Starch—all glucose).
- Nucleic Acids: Heteropolymers (A, T, G, C).


